Phenols
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH). Mildly acidic, it requires careful handling because it can cause chemical burns.
Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs.

Tests for Phenolic [Ar—OH] Group
The phenolic group can be detected by the following tests:
- Litmus test
- Ferric chloride test
- Libermann’s test
- Bromine water test
- Phthalein dye test
Materials Required
- Blue litmus paper
- Ferric chloride solution
- Sodium nitrite
- Concentrated sulfuric acid
- Sodium hydroxide
- Bromine water
- Phthalic anhydride
- Organic compound to be tested
- Test tubes
- Test tube holder
- Dropper
- Beaker
Litmus Test
In laboratory, a litmus paper is used to test whether the given solution is acidic or basic. Red litmus paper turns blue while blue litmus paper remains unchanged in the presence of a base.
Procedure
- Place the drop of given organic solution or a small crystal on moist blue litmus paper.
- Observe the change in colour, if it changes to red then phenolic group may be present.
Phenol turns blue litmus paper red. This shows that phenol is acidic in nature. Carboxylic acid also give this test. Compare to carboxylic acid phenol is weakly acidic and it does not give an effervescence with aqueous sodium carbonate.
Ferric Chloride Test
Aqueous solution of phenol reacts with freshly prepared ferric chloride solution gives coloured complex. Most phenols give dark coloured solution.Ferric chloride solution: Neutral solution of ferric chloride is prepared by adding diluted solution of sodium hydroxide to ferric chloride solution drop by drop until a small but permanent brown precipitate appears. Filter the solution and use the clear filtrate for the test.
Here is the chemical reaction

Procedure
- Dissolve the given organic compounds in water.
- Add neutral solution of ferric chloride slowly dropwise.
- Observe the change in colour.
- A red, blue, green or purple colouration indicates the presence of phenol.
Note:
1.o, m, p-cresol, resorcinol give violet or blue colouration.
2. β-Naphthol gives a green colouration.
3. α-Naphthol gives pink colouration.
4. Formic acid and acetic acid give deep red colouration.
Liebermann’s Test
Phenol reacts with concentrated sulfuric acid and sodium nitrite forms a yellow colour quinone monoxime complex. With excess of phenol and sulfuric acid a deep blue indophenol complex is formed. On dilution a red colour indophenol is formed which turns to deep blue colour sodium salt solution of indophenol on treatment with sodium hydroxide.


Procedure
- Place the crystals of sodium nitrite in a clean dry test tube.
- Add 1ml of phenol to sodium nitrite solution.
- Heat the mixture gently and allow it to cool.
- Add 1ml of concentrated sulfuric acid to it and shake the contents.
- Observe the change in the colour of the solution.
- Dilute the solution with water so that the given compound turns red if phenolic group is present.
- Now add sodium hydroxide solution, the blue colour solution or green colour solution appears.
Note:
1. Nitrophenols and p-substituted phenols do not give this test.
2. Among the dihydroxyphenols, only resorcinol gives positive test.
Phthalein Dye Test
Phenol on heating with phthalic anhydride in the presence of concentrated sulfuric acid forms a colourless condensation compound called phenolphthalein. On further reaction with dilute sodium hydroxide solution gives a pink colour fluorescent compound called fluorescein. Characteristic colours are produced by different phenolic compounds which can be viewed under white background.
Here is the chemical reaction:
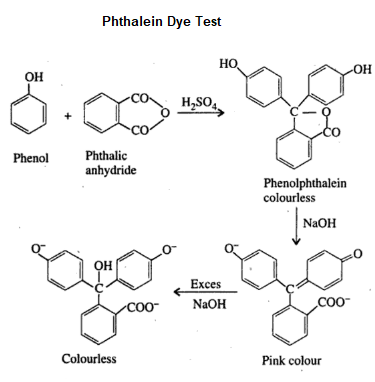
Procedure
- Take the organic compound to be tested in a test tube.
- Add 200mg of phthalic anhydride to it.
- Add drops of concentrated sulfuric acid to the mixture.
- Heat the solution for 2-3 minutes.
- Cool the mixture and pour it into a beaker containing dilute sodium hydroxide solution.
- Dilute the whole mixture with equal volume of water.
- Observe the change in the colour in a white background.
- If fluorescence colour exists the view it in a black background.
The colours produced by different phenolic compounds in phthalein dye test is given below
| Phenol | Reddish pink |
| o-cresol | Red |
| m-cresol | blue or violet blue |
| 1-naphthol | green |
| 2-naphthol | faint green |
| Resorcinol | yellow-green fluorescence |
| Hydroquinone | deep purple |
Bromine Water Test
Phenol undergoes electrophilic substitution reaction with bromine. When bromine water is added to aqueous solution of phenol the brown colour of bromine disappears and a white precipitate of tribromophenol is formed.
Here is the chemical reaction
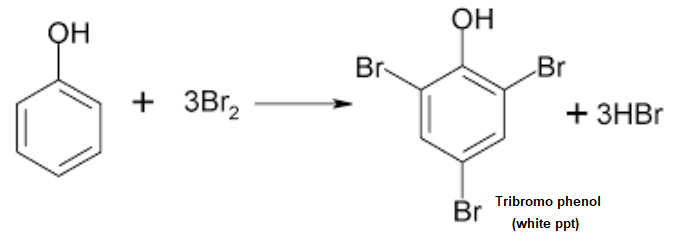
Procedure
- Take 5ml of bromine add 100ml of distilled water and shake well. Decant off the clear liquid.
- Dissolve the given organic compound in glacial acetic acid.
- Add bromine water solution to this dropwise.
- If the colour of bromine disappears then it indicates the presence of phenol.
Uses Of Phenols
- Phenol is used as an antiseptic and disincentive agent.
- Phenol has been used in many cosmetics preparations such as sun screens, hair colorings and skin formulations.
- Longer alcohols and fatty alcohols have been used in the industries of plasticizers and detergents.
- Some phenolic compounds are used in food additives.
- Phenolic compounds are used as drugs and prodrugs for treatments of several diseases such as Parkinson’s disease.
- Some phenols are used in chemical synthesis procedures or as indicators.
Summary of Tests For Phenol Groups
| Litmus test | Phenol turns blue litmus paper red. |
| Ferric chloride test | Violet or blue colouration shows presence of phenol. |
| Libermann’s test | Deep blue colour solution shows presence of phenol. |
| Bromine water test | Formation of white precipitate shows presence of phenol. |
| Phthalein dye test | Pink colour solution shows presence of phenol. |