Carboxylic Acids
Carboxylic acid, any of a class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (―OH) by a single bond. A fourth bond links the carbon atom to a hydrogen (H) atom or to some other univalent combining group. The carboxyl (COOH) group is so-named because of the carbonyl group (C=O) and hydroxyl group.
The chief chemical characteristic of the carboxylic acids is their acidity. They are generally more acidic than other organic compounds containing hydroxyl groups but are generally weaker than the familiar mineral acids (e.g., hydrochloric acid, HCl, sulfuric acid, H2SO4, etc.).
Carboxylic acids occur widely in nature. The fatty acids are components of glycerides, which in turn are components of fat. Hydroxyl acids, such as lactic acid (found in sour-milk products) and citric acid (found in citrus fruits), and many keto acids are important metabolic products that exist in most living cells. Proteins are made up of amino acids, which also contain carboxyl groups.
Compounds in which the ―OH of the carboxyl group is replaced by certain other groups are called carboxylic acid derivatives, the most important of which are acyl halides, acid anhydrides, esters, and amides.
Carboxylic acids can be identified by the following tests:
- Litmus test.
- Sodium bicarbonate test.
- Ester test.
- Fluorescein test
Materials Required
- Blue litmus paper
- Sodium bicarbonate (or) sodium hydrogen carbonate
- Ethyl alcohol
- Concentrated sulfuric acid
- Resorcinol
- Acid anhydride
- Given organic compound
- Test tubes
- Test tube holders
- Beaker
- Glass rod
- Stirrer
Litmus Test
The carbobxylic acids turn blue litmus red. The hydroxyl group in —COOH is far more acidic than in alcohol.

- Add a drop of given organic compound on blue litmus paper.
- Observe the colour change in blue litmus paper.
- If the colour of blue litmus changes to red the presence of carboxylic acid.
Note: Blue litmus solution is also used in the place of blue litmus paper.If the colour of the blue litmus paper changes to red then carboxylic group is present. Phenol also gives this test.
Sodium Bicarbonate Test
Carboxylic acids react with sodium hydrogen carbonate to give carbon dioxide gas which is identified by the effervescence produced. This test is used to distinguish carboxylic acids from phenols.

- Prepare a saturated solution of sodium bicarbonate by dissolving sodium bicarbonate in 1ml of water.
- Add the given organic compound on the saturated solution of sodium bicarbonate solution.
- Shake the solution well.
- If there is an evolution of brisk effervescence then it indicates the presence of carboxylic acid.
Note: Use acid free alcohol for the test.
Ester Test
A carboxylic acid reacts with an alcohol in presence of a little sulphuric acid to form an ester which is recognized by its fruity smell.

Procedure
- Mix the given compound with ethyl alcohol and concentrated sulfuric acid.
- Heat the mixture in a dry test tube in a water bath.
- Pour the reaction mixture into a beaker carefully containing water.
- Neutralise the excess sulfuric acid.
- If a sweet smelling substance is sensed then it indicates the presence of acid.
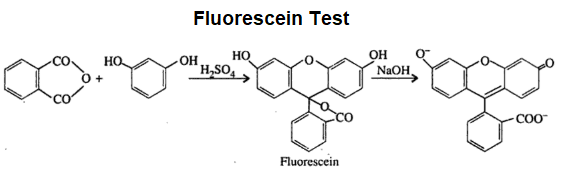
Fluorescein Test
This test is given by dicarboxylic acid. Dicarboxylic acid on heating gives acid anhydride. When this anhydride is treated with resorcinol in the presence of concentrated sulfuric acid a fluorescent
dye is formed and so this reaction is called fluorescein test.
The chemical reaction is given below.
Note: This test should be performed only if the compound gives positive results in litmus test and sodium bicarbonate test.