Isobutylbenzene, also known as 2-methyl-1-phenylpropane, is an organic compound with the chemical formula C10H14. It is classified as an alkylbenzene, which means it is derived from benzene by replacing one of the hydrogen atoms with an alkyl group. In the case of isobutylbenzene, the alkyl group is an isobutyl group (a branched chain of four carbon atoms). Isobutylbenzene is a colorless flammable liquid that is a respiratory irritant.
Chemical Structure
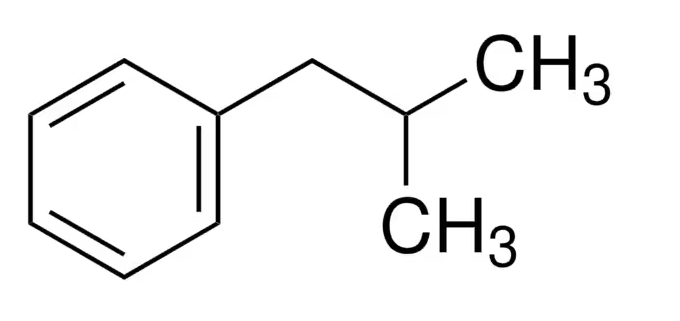
Isobutylbenzene has a molecular formula of C10H14. Its chemical structure consists of a benzene ring (C6H5) attached to a side chain composed of three carbon atoms (C3H7), one of which is substituted with a methyl group (CH3).
- The benzene ring, which consists of six carbon atoms arranged in a hexagonal ring, forms the core structure of isobutylbenzene. Each carbon atom in the benzene ring is bonded to one hydrogen atom.
- Attached to the benzene ring is a side chain consisting of three carbon atoms. This side chain is derived from butane (C4H10) by removing one hydrogen atom.
- One of the carbon atoms in the side chain is directly bonded to the benzene ring. This carbon atom is also bonded to two other carbon atoms and one hydrogen atom, forming the isobutyl group (CH3-CH-CH2-).
- One of the carbon atoms in the isobutyl group is further substituted with a methyl group (CH3), resulting in the methyl branch.
Physical Properties of Isobutylbenzene
- Physical State: It is typically a colorless to pale yellow liquid at room temperature.
- Odor: It has a characteristic aromatic smell.
- Density: The compound has a density of about 0.864 g/cm^3 at 25°C.
- Melting Point: Its melting point is around -81°C.
- Boiling Point: The boiling point is approximately 170-172°C.
- Solubility: It is insoluble in water but soluble in organic solvents such as ethanol, diethyl ether, and acetone.
- Vapor Pressure: At 25°C, the vapor pressure is about 3.5 mmHg.
- Viscosity: It has a relatively low viscosity compared to water.
- Refractive Index: The refractive index is typically around 1.495 at 20°C.
- Flash Point: The compound has a flash point above 37.8°C (100°F), making it flammable under certain conditions.
Chemical Properties of Isobutylbenzene
- Aromatic Substitution: It can undergo electrophilic aromatic substitution reactions, where a hydrogen atom on the benzene ring is replaced by a functional group or another atom.
- Halogenation: It can undergo halogenation reactions, such as chlorination or bromination, in the presence of a halogen and a suitable catalyst.
- Nitration: It can be nitrated to produce nitro derivatives, using a mixture of nitric and sulfuric acids as the nitrating agent.
- Sulfonation: It can undergo sulfonation reactions, where a sulfonic acid group (-SO3H) is introduced onto the benzene ring, using concentrated sulfuric acid as the sulfonating agent.
- Friedel-Crafts Acylation and Alkylation: It can participate in Friedel-Crafts reactions, where acyl or alkyl groups are added to the benzene ring in the presence of a Lewis acid catalyst, such as aluminum chloride.
- Oxidation: It can be oxidized to form various products, depending on the conditions and reagents used. For example, oxidation with chromic acid can lead to the formation of carboxylic acids.
- Hydrogenation: It can undergo hydrogenation reactions, where hydrogen gas is added across the double bonds in the molecule, in the presence of a metal catalyst such as platinum or palladium.
- Esterification: It can undergo esterification reactions with carboxylic acids to form esters, in the presence of an acid catalyst.
- Aromatic Ring Cleavage: Under certain conditions, such as high temperature and pressure, it can undergo aromatic ring cleavage reactions and result to the formation of smaller aromatic compounds or aliphatic products.
Uses of Isobutylbenzene
- It is used as a solvent in industrial applications, such as in the manufacturing of paints, coatings, varnishes, adhesives, and printing inks.
- It is used in the industrial manufacture of ibuprofen.
- It serves as a medium for dissolving and dispersing other substances.
- Isobutylbenzene can be used as a fragrance ingredient in perfumes, colognes, and other cosmetic products.
- It is used in the rubber industry as a processing aid and solvent for rubber compounds.
- It can be found in some cleaning products, such as degreasers and solvent-based cleaners, for removing oils, greases, and other contaminants.
- It is sometimes used as a fuel additive in gasoline to improve its octane rating and combustion characteristics.
- Some formulations of insecticides and pesticides may contain isobutylbenzene as an active ingredient or solvent.