Succinic acid, also known as butanedioic acid, is a dicarboxylic acid with the chemical formula C4H6O4. It is a colorless crystalline solid with a sour taste and is soluble in water. Succinic acid occurs naturally in various plants and animal tissues, as well as in some microorganisms. It is an intermediate in several metabolic pathways in living organisms.
When it was first discovered, it was extracted from amber by pulverizing and distilling it using a sand bath. It was primarily used externally for rheumatic aches and pains.
Chemical Structure
It has a molecular formula of C4H6O4. Its chemical structure consists of a four-carbon chain with two carboxylic acid groups (-COOH) attached at the ends.
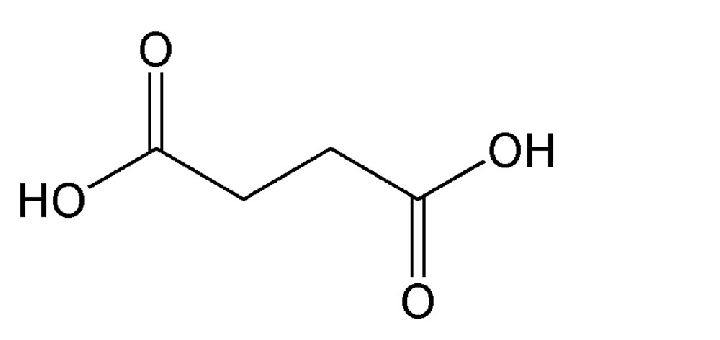
In this structure:
- The four carbon atoms are arranged in a straight chain.
- Each of the two terminal carbon atoms is bonded to a carboxylic acid group (-COOH).
- The carbon atoms in the middle of the chain are each bonded to two hydrogen atoms (-CH2-) and are connected by a single bond.
- The carbon atoms at the ends of the chain are bonded to one oxygen atom from the carboxylic acid group and another carbon atom from the chain.
The presence of two carboxylic acid groups gives succinic acid its acidic properties. These groups can ionize in aqueous solutions, releasing hydrogen ions (H+) and forming succinate ions (CH2(COO)2-) which contributes to its sour taste and ability to act as an acidity regulator.
Physical Properties of Succinic Acid
- Physical State: Solid at room temperature
- Molecular Weight: Approximately 118.09 g/mol
- Color: Colorless or white
- Odor: Odorless
- Taste: Sour taste
- Solubility: Soluble in water, ethanol, and ether
- Hygroscopicity: Slightly hygroscopic
- Melting Point: 185-187°C
- Boiling Point: Decomposes before boiling
- Density: 1.563 g/cm^3 (solid)
- pKa: pKa1 = 4.20, pKa2 = 5.64 (related to its two carboxylic acid groups)
- Crystalline Structure: Forms crystalline structures
- Appearance: Forms transparent to translucent crystals
- Safety: Generally recognized as safe (GRAS) by regulatory agencies when used in accordance with good manufacturing practices (GMP)
- Storage: Store in a cool, dry place away from heat and moisture
Chemical Properties of Succinic Acid
- Succinic acid is a dicarboxylic acid, containing two carboxylic acid functional groups (-COOH).
- It acts as a dibasic acid due to the presence of two carboxylic acid groups, meaning it can donate two protons (H⁺ ions) in acid-base reactions.
- Can be produced from renewable resources such as biomass
- Undergoes esterification reactions with alcohols to form esters.
- Can undergo dehydration reactions to form cyclic anhydrides (succinic anhydride).
- Reacts with bases to form salts known as succinates.
- Undergoes oxidation reactions to form various oxidation products.
- Can participate in condensation reactions with other compounds, particularly compounds containing amino groups (amides and peptides).
- Reacts with reducing agents to undergo reduction reactions.
- Can undergo decarboxylation reactions under certain conditions.
- May form coordination complexes with metal ions, acting as a bidentate ligand.
- Exhibits acidity due to the dissociation of its carboxylic acid groups in aqueous solutions.
- Can undergo substitution reactions on its alkyl or functional groups under appropriate conditions.
Preparation of Succinic Acid
Succinic acid can be prepared through both synthetic chemical routes and biological processes.
- Petroleum-Based Synthesis: Historically, succinic acid was primarily produced through chemical synthesis from petroleum-derived feedstocks. One common method involves the catalytic hydrogenation of maleic acid or maleic anhydride, which yields succinic acid. This process employs catalysts such as nickel or palladium under high-pressure conditions.
- Butane Oxidation: This involves oxidation of n-butane or maleic anhydride with oxygen or air. This method requires the use of catalysts such as vanadium pentoxide or cobalt-manganese oxides. The oxidation of butane yields maleic acid, which can then be hydrogenated to produce succinic acid.
- Bio-Based Production: With increasing interest in sustainable and renewable processes, there has been a growing focus on the biological production of succinic acid. This can be achieved through fermentation processes using certain microorganisms, particularly bacteria such as Actinobacillus succinogenes, Mannheimia succiniciproducens, and Escherichia coli. These bacteria can ferment different carbon sources like glucose, xylose, glycerol, and other renewable feedstocks, to produce succinic acid as a fermentation product.
- Electrochemical Synthesis: This approach involves the electrolysis of maleic acid or maleate salts in an electrolytic cell to produce succinic acid.
- Biocatalytic Routes: Enzymatic processes using enzymes such as succinate dehydrogenase or fumarase have been investigated for the conversion of certain substrates to succinic acid. These enzymatic routes offer high selectivity and mild reaction conditions but may face challenges related to enzyme stability and substrate specificity.
Uses of Succinic Acid
- Included in skincare products and cosmetics as a moisturizing and skin-conditioning agent.
- Used as an acidity regulator and flavor enhancer in food and beverage products.
- Used as an excipient in drug formulations, especially in controlled-release formulations.
- Used in the production of succinate-based medications for the treatment of metabolic disorders.
- Used as a pH adjuster and buffering agent in cosmetic formulations.
- Used as a monomer in the production of biodegradable polymers such as polybutylene succinate (PBS) and polybutylene succinate adipate (PBSA).
- Used in the production of specialty chemicals and fine chemicals.
- Succinic acid is also used as an antibiotic and curative agent.
- Serves as a component in the production of biodegradable batteries.
- Used in textile dyeing and finishing processes as a pH regulator and dye fixative.
- Added to industrial cleaning products as a chelating agent and pH adjuster.