Trinitrosotrimethylenetriamine, also known as RDX, is an explosive compound with the molecular formula C3H6N6O6. It is a white crystalline solid. It is commonly used in military and industrial applications because of its high explosive power. RDX is known for its high detonation velocity and sensitivity to heat, shock, and friction.
RDX is produced by reacting hexamine with nitric acid and ammonium nitrate in the presence of sulfuric acid. The reaction is carried out in a controlled environment to ensure safety and prevent accidents.
In terms of properties, RDX has a melting point of around 204-205°C and a density of 1.82 g/cm3 at 20°C. It is insoluble in most organic solvents and only slightly soluble in water. The vapor pressure of RDX is negligible, and it is relatively stable under normal conditions but can decompose under heat or shock.
Structure
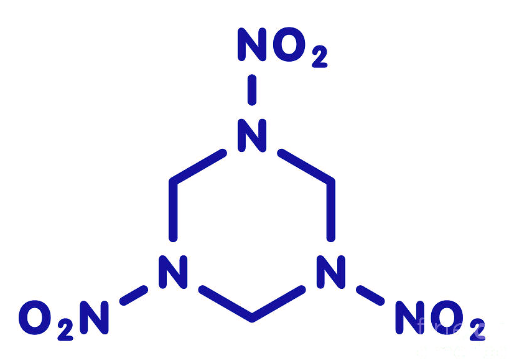
RDX is a nitramine explosive compound with a six-membered ring structure composed of three nitrogen and three carbon atoms.
In its chemical structure, RDX has a symmetrical arrangement of three nitro groups (-NO2) attached to the ring. Each carbon atom is bonded to two hydrogen atoms and one nitrogen atom, while each nitrogen atom is bonded to one carbon atom and either one or two hydrogen atoms. The remaining three bonds of the nitrogen atoms are occupied by the nitro groups.
Properties
| Characteristic | Description |
|---|---|
| Chemical Formula | C₃H₆N₆O₆ |
| Molecular Weight | Approximately 222.10 g/mol |
| Appearance | White crystalline solid |
| Melting Point | 204-205°C |
| Boiling Point | Decomposes before boiling |
| Density | 1.82 g/cm³ (at 20°C) |
| Solubility | Slightly soluble in water, insoluble in most organic solvents |
| Vapor Pressure | Negligible |
| Stability | Relatively stable under normal conditions, but can decompose under heat or shock |
| Explosive Properties | High detonation velocity, sensitive to heat, shock, and friction |
Preparation of Trinitrosotrimethylenetriamine (RDX)
Setup and Safety Precautions
- Perform the reaction in a well-ventilated fume hood to minimize exposure to hazardous fumes.
- Use appropriate personal protective equipment (PPE), including gloves, goggles, and a lab coat.
- Ensure all chemicals are stored and handled according to safety regulations and guidelines.
Reagents Preparation
- Dissolve 7 g of hexamethylenetetramine (hexamine) in 200 mL of ice-cold water to ensure complete dissolution.
- Prepare a solution containing 15 g of sodium nitrite dissolved in 50 mL of water. Ensure the solution is thoroughly mixed and free of any undissolved solids.
- Prepare 6 N hydrochloric acid solution and adjust the pH to 1 using pH paper or a pH meter.
Reaction Procedure
- Place the solution of hexamine in a suitable reaction vessel, preferably equipped with a stirring mechanism and a temperature control system.
- With constant stirring, slowly add the solution containing sodium nitrite and 6 N hydrochloric acid dropwise to the hexamine solution to maintain the pH around 1.
- Maintain the reaction temperature at 0°C using an ice bath or a controlled cooling system.
- Allow the reaction mixture to stir at 0°C for at least 30 minutes to ensure complete reaction.
Product Isolation
- After the reaction is complete, carefully collect the precipitated trinitrosotrimethylenetriamine (RDX) by filtration using a suitable filter paper or glass filter funnel.
- Wash the collected RDX with small portions of ice-cold water to remove any impurities or unreacted starting materials.
- Dry the washed RDX thoroughly under reduced pressure or in a desiccator to remove excess water.
Product Characterization
- Determine the yield of the isolated RDX by weighing the dried product.
- Confirm the purity of the RDX product using analytical techniques such as melting point determination, infrared spectroscopy (IR), and nuclear magnetic resonance (NMR) spectroscopy.
References
- A. S. Kumar, V. B. Rao, and R. K. Sinha, “Evaluation of plastic bonded explosive (PBX) formulations based on RDX, aluminum, and HTPB for underwater applications,” Propellants Explosives Pyrotechnics, vol. 35, no. 4, pp. 359–364, 2010.View at: Publisher Site | Google Scholar
- P. P. Vadhe, R. B. Pawar, and R. K. Sinha, “Cast aluminized explosives (review),” Combustion Explosion & Shock Waves, vol. 44, no. 4, pp. 461–477, 2008.View at: Publisher Site | Google Scholar
- X. H. Li, H. B. Pei, and X. Zhang, “Effect of aluminum particle size on the performance of aluminized explosives,” Propellants Explosives Pyrotechnics, vol. 45, no. 5, pp. 807–813, 2020.View at: Publisher Site | Google Scholar
- A. N. Zhigach, I. O. Leipunskii, and N. G. Berezkina, “Aluminized nitramine-based nanocomposites: manufacturing technique and structure study,” Combustion Explosion & Shock Waves, vol. 45, no. 6, pp. 666–677, 2009.View at: Publisher Site | Google Scholar