What Is Ninhydrin Test?
The ninhydrin test is a chemical test which is used to check whether a given analyte contains amines or alpha-amino acids. In this test, the main reactant is ninhydrin which is a hydrocarbon with a chemical compound with the formula C9H6O4. By IUPAC nomenclature standards name, ninhydrin is also referred to as 2,2-dihydroxyindane-1,3-dione. When ninhydrin is added to a test solution of the analyte, there is development of a deep blue color which indicates the presence of ammonia, primary/secondary amines, or amino acids in the analyte.
Ninhydrin Test Objective
- To detect the presence of amines and amino groups in the test solution.
- To quantify the amino acids present in the sample.
- To distinguish carbohydrates from amino acids.
Principle Of Ninhydrin Test
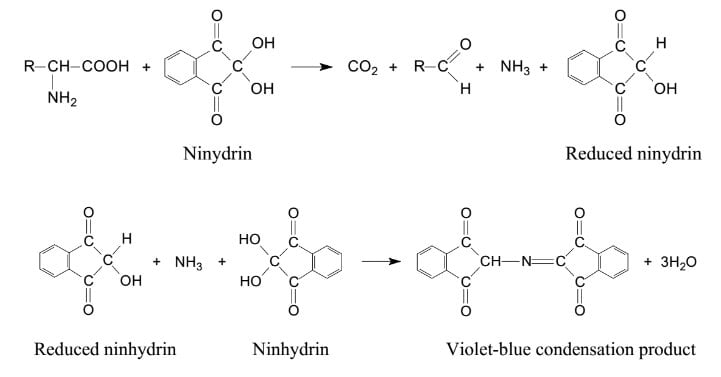
The amino group belonging to a free amino acid undergoes a chemical reaction with ninhydrin, which act as an oxidizing agent. When exposed to ninhydrin, the amino acid undergoes oxidative deamination, resulting in the liberation of CO2, NH3, and an aldehyde along with hydrindantin (which is a reduced form of ninhydrin). Ammonia further reacts with another ninhydrin molecule to form diketohydrin (which is also known as Ruhemann’s complex). This complex is responsible for the deep blue color. In amino acids like proline and hydroxyproline, this test yields an iminium salt, which is yellow-orange in color. Similarly, proteins with a free amind group like asparagine, react with the ninhydrin reagent to form a brown colored product. The intensity of the formed complex is proportional to the concentration of amino acids in the solution. The color intensity, in turn, depends on the type of amino acid present.
Experiment
Reagents And Material Required
Reagent
- 2% Ninhydrin reagent: 2% solution of ninhydrin is prepared by dissolving 0.2 grams of ninhydrin in 10ml of either ethanol or acetone.
- Diluent solvent (for the quantitative test): Mix equal volumes of water and n-propanol.
- Standard solution (1% protein solution)
- Sample solution
Material Required
- Test tubes
- Test tube stand
- Pipette
- Water bath
- Spectrophotometer
Ninhydrin Test Procedure
For Qualitative Analysis
- Take 1 ml of standard protein solution in one test tube and 1 ml of the test sample in another dry test tube.
- Add a few drops of ninhydrin reagent to both the test tubes.
- Place the test tubes in the water bath for 5 minutes and then allow cooling to room temperature.
- Observe the formation of color and note down the result.
For Quantitative Analysis
- Pipette out different volumes (50 µl, 100 µl, and so on) of protein solution from the supplied stock solution (200µg /ml) into a series of test tubes and make up the volume to 1 mL with distilled water.
- Take a tube labeled as one as blank containing 1ml of just distilled water and the rest of the tubes labeled 2 to 9 for construction of a standard curve. Tubes 10-15 are for the unknown samples.
- Add 2 ml of the ninhydrin reagent and 5ml of diluent solvent to each tube and mix well by vortexing.
- Cool the tubes.
- Cover the tubes with caps on top and incubate at 90°C for 17 minutes or boiling water bath for 15 minutes.
- Cool the tubes to room temperature and measure the optical density of the solutions at 570 nm (440 nm for proline and hydroxyproline) against a blank.
- Prepare a standard curve of absorbance against amino acid concentration.
- Determine the amount of amino acid in the unknown sample by constructing a standard calibration curve on a graph paper, by plotting the amino acid concentration (10 to 100mg) on x-axis and absorbance at 570 nm on the y-axis.
Note: Cool the contents of all the tubes on ice before adding ice-cold anthrone reagent.
Ninhydrin Test Result Interpretation
- For ammonia, primary/secondary amines, and amino acids, deep purple colour is obtained.
- For hydroxyproline and proline, a yellow colour is obtained.
- For asparagine, brown colour is obtained.
- If no colour change is observed, the analyte does not contain amino acids, amines, or ammonia.
- From the graph, we can determine the concentration of unknown samples.
Calculation
- Amount of amino acid present in 100mg of the sample =(mg of protein ÷ Volume of test sample) X 100
Uses Of Ninhydrin Test
- It is helpful in monitoring deprotection in solid phase peptide synthesis. If nitrogen is deprotected, the ninhydrin test turns blue.
- It is used in the analysis of amino acids in proteins. Most amino acids hydrolyzed and react with ninhydrin with the exception of proline. Some amino acid chains degrade. Therefore, a separate analysis is needed to identify amino acids that may react or not react with ninhydrin.
- It is used to verify a solution suspected of having ammonium ions. A treatment with ninhydrin would result in a dramatic purple color.
- It is often used by forensic investigators in the analysis of fingerprints on porous surfaces. A finger mark contains amino acids is treated with ninhydrin solution, which results in a purple amino acid finger crest pattern. Therefore making the fingerprint visible.
Limitations of Ninhydrin Test
- Ninhydrin reacts not only reacts with α-amino groups but also with nitrogen in ammonia and other free amines.
- The Ninhydrin test is not effective to detect high molecular weight proteins as the steric hindrance limits the ninhydrin from reaching the α-amino groups.
What You Need To Know About Ninhydrin Test
- Many bioanalytical procedures use ninhydrin, especially for amino acid analysis method.
- Ninhydrin test is used by SSDs for residual protection detection on re-usable surgical instruments.
- Amino acids react with ninhydrin, which results in discoloration.
- Ninhydrin test is used in both quantitative and qualitative purposes – such as chromatographic visualization and peptide sequencing.
- Bluish to purplish discoloration is produced by the a-amino acids while yellow to orange discoloration is caused by secondary amine like proline.
- Ninhydrin test is extremely sensitive that it can be used to visualize fingerprints.
- Ninhydrin is the most preferred chemical for the visualization of fingerprints on porous materials and paper as it reacts with the amino acids in the sweat left behind in a fingerprint.
- The strong compound formed by ninhydrin is called Ruhemann’s purple.
- The strongly colored compound that is then formed is called Ruhemann’s purple.
- Ninhydrin reacts with compounds containing amine such as proteins in the blood.