A scientist named Lewis gave the electron dot structure of molecules which are helpful in better understanding of the bonding and non-bonding electrons present in a molecule. Water, one of the Earth’s major constituents, has the molecular formula H2O. A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond.
Molecular geometry of water
The molecular geometry of water is bent or angular. This means that the H-O-H bond angle is approximately 104.5 degrees. This bent shape arises due to the presence of two lone pairs of electrons on the oxygen atom, which repel the bonded pairs, causing the two hydrogen atoms to be pushed closer together. This geometry gives water its unique properties, such as its ability to form hydrogen bonds and its high polarity, which are essential for its role as a universal solvent.
Lewis Structure of H2O
The Lewis structure of a molecule is a diagram that shows the arrangement of atoms and valence electrons within the molecule. For water (H2O), a Lewis structure helps us understand the bonding and electron distribution in the molecule.
In the Lewis structure of water, we have two hydrogen atoms (H) bonded to a central oxygen atom (O). Oxygen has six valence electrons, and hydrogen has one valence electron each. Therefore, the total number of valence electrons in water is:
2 (hydrogen atoms) × 1 electron each + 6 (oxygen atom) = 8 electrons
This means there are 4 electron pairs available for bonding. In the Lewis structure, these electrons are represented by dots around the symbol for each atom, or by dashes representing shared pairs of electrons in covalent bonds.
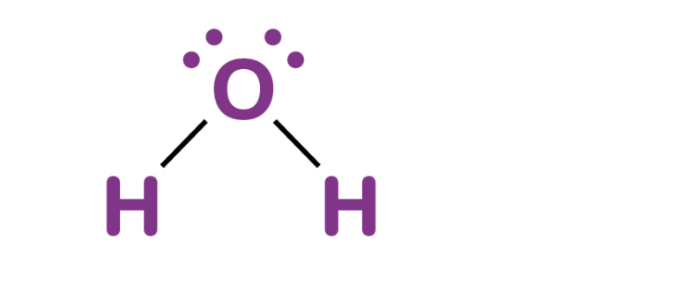
Drawing the Lewis structure of water (H2O)
Drawing the Lewis structure of water (H2O) involves representing the valence electrons of the hydrogen and oxygen atoms and arranging them to form covalent bonds between the atoms.
Step-by-step guide:
- Count the total number of valence electrons: Hydrogen (H) has 1 valence electron, and oxygen (O) has 6 valence electrons. Since there are two hydrogen atoms in water, the total number of valence electrons is 2 (for hydrogen) + 6 (for oxygen) = 8 electrons.
- Determine the central atom: In water, oxygen is the central atom because hydrogen can only form one bond, while oxygen can form two.
- Connect the atoms with single bonds: Oxygen forms single bonds with each hydrogen atom. Each bond consists of 2 electrons, so 2 electrons are used to form the bond between oxygen and each hydrogen.
- Distribute the remaining electrons: After forming the bonds, distribute the remaining electrons around the atoms to satisfy the octet rule (except for hydrogen, which follows the duet rule). Oxygen needs 8 electrons (including the shared ones) to satisfy the octet rule.
- Place lone pairs: If there are remaining electrons after forming the bonds, place them as lone pairs on the central atom (oxygen) until it satisfies the octet rule.
In other words:
- Hydrogen has 2 electrons (1 valence electron each), and oxygen has 6 electrons (6 valence electrons). So, we have 8 valence electrons in total.
- Oxygen is the central atom because it can form more bonds.
- Connect each hydrogen atom to the oxygen atom with single bonds. This uses 4 electrons, leaving 4 more.
- Distribute the remaining 4 electrons as lone pairs on the oxygen atom.