What Is Anthrone Test?
Anthrone is a tricyclic sweet-smelling ketone. It is utilized for a well-known cellulose measure and in the colorimetric determination of carbohydrates. Anthrone test is used for qualitative and quantitative estimation of polysaccharides as well as monosaccharides. In this test, carbohydrates are dehydrated with concentrated sulphuric acid (H2SO4) to form ‘’furfural’’, which condenses with anthrone to form a green color complex which can be measured colorimetrically at 620nm or by using a red filter.
Objective
- To detect the presence of carbohydrate in a given sample.
Principle of Anthrone Test
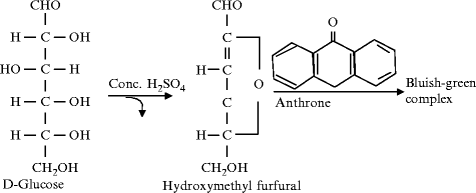
Anthrone test principle is the same as molisch’s test principle but here anthrone reagent is used instead of molisch’s reagent. Carbohydrates are first hydrolysed into simple sugars by concentrated sulphuric acid. In hot acid medium, glucose is dehydrated to hydroxylmethyl furfural. The hydroxylmethyl furfural formed condenses with two molecules of naphthol from the anthrone reagent to form a blue-green colored complex. The complex can be quantified by measuring the absorbance of 620 nm wavelength in a spectrophotometer or in a red filter colorimeter.
Experiment
Reagent And Material Required
Reagent
- Anthrone reagent
- Glucose
- Other carbohydrates if desired
Material required
- UV spectrophotometer
- Vortex mixer
- Mantle heater/water bath
- Test tubes
- Test tube stand
- Pipette
- Beaker
- Tissue paper
- Wash bottle
Reagent Preparation
- Anthrone reagent: Dissolve 200mg of anthrone reagent in 100ml of concentrated H2SO4.
- Standard Glucose solution: a) Stock standard: Weigh 100mg of Glucose and transfer it carefully into a 100ml withDistilled water. (100mg of Glucose in 100ml of Distilled water). b) Working standard: Dilute 10ml of stock standard solution in 100ml with distilled water in a volumetric flask.
Anthrone Test Procedure
- Pipette out different volumes (50 µl, 100 µl, and so on) of glucose solution from the supplied stock solution (200µg /ml) into a series of test tubes and make up the volume to 1 mL with distilled water.
- Take a tube labeled as one as blank containing 1ml of just distilled water and the rest of the tubes labeled 2 to 9 for construction of a standard curve. Tubes 10-15 are for the unknown samples.
- Add 5 ml of the anthrone reagent to each tube and mix well by vortexing.
- Cool the tubes.
- Cover the tubes with caps on top and incubate at 90°C for 17 minutes or boiling water bath for 10 minutes.
- Cool the tubes to room temperature and measure the optical density of the solutions at 620 nm against a blank.
- Prepare a standard curve of absorbance against glucose concentration.
- Determine the amount of glucose in the unknown sample by constructing a calibration curve on a graph paper, by plotting the glucose concentration (10 to 100mg) on x-axis and absorbance at 620nm on the y-axis.
Note: Cool the contents of all the tubes on ice before adding ice-cold anthrone reagent.
Anthrone Test Result Interpretation
- The presence of a blue-green complex indicates the presence of carbohydrates in the given solution.
- From the graph, we can determine the concentration of unknown samples.
Calculation
- Amount of carbohydrate present in 100mg of the sample =(mg of glucose ÷ Volume of test sample) X 100
Uses of Anthrone Test
- Anthrone test is used for the detection and quantification of carbohydrates in various samples like blood serum, milk, and its variation, etc.
Limitations of Anthrone Test
- Anthrone test gives a negative result in samples that are carbohydrates like D-glucose phenylosazone and D-glucose phenylosotriaole but do not form furfural or hydroxyfurfural in the dehydration step.