Maleic anhydride is an organic compound with the formula C₂H₂(CO)₂O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Structure
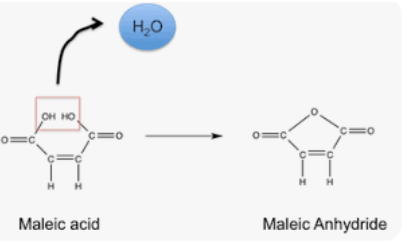
The structure of maleic acid contains two carboxylic acid functional groups, which classifies maleic acid as a dicarboxylic acid functional group. Functional group is defined as the specific group of atoms or bonds responsible for a compound’s properties. Some properties of the carboxyl functional group are a high melting point and boiling point compared to substances with the same molar mass.
Physical Properties of Maleic anhydride
- Chemical formula: C4H2O3
- Molecular weight: 98.06 g/mol
- Appearance: Colorless to white solid
- Odor: Acrid
- Melting point: 52.8 °C (127.0 °F)
- Boiling point: 202 °C (396 °F)
- Density: 1.48 g/cm3 (solid)
- Solubility: Soluble in water, alcohol, acetone, and ether
- Reactivity: Maleic anhydride is highly reactive and can undergo addition reactions with nucleophiles, such as alcohols and amines, to form various products.
- Stability: It is relatively stable under normal conditions but can undergo hydrolysis in the presence of water to form maleic acid.
- Toxicity: Maleic anhydride can be irritating to the eyes, skin, and respiratory system. It should be handled with care and appropriate safety precautions should be taken.
Chemical Properties of Maleic anhydride
- It is an organic compound belonging to the class of cyclic anhydrides.
- It is highly reactive due to the presence of the anhydride functional group, which consists of two carbonyl groups bonded to each other.
- Readily undergoes addition reactions with nucleophiles, such as alcohols and amines, to form corresponding addition products.
- In the presence of water or hydroxide ions, maleic anhydride undergoes hydrolysis to form maleic acid.
- It can be used as a dienophile in Diels-Alder reactions to form cyclic compounds.
- It is used as a precursor in the production of various chemicals, including maleic acid, fumaric acid, and various resins.
- Under certain conditions, maleic anhydride can undergo polymerization to form polymers such as poly(maleic anhydride) or copolymers with other monomers.
- It is susceptible to oxidation reactions under certain conditions.
- It act as a cross-linking agent in the production of certain polymers and enhancing their mechanical properties.
Preparation of Maleic Anhydride
Maleic anhydride can be prepared through several methods, but one of the most common industrial processes involves the oxidation of benzene or n-butane.
Benzene Oxidation
- Benzene can be oxidized to maleic anhydride via a catalytic process using air or oxygen as the oxidizing agent. This process usually involves a multi-step reaction sequence.
- In the first step, benzene is converted to maleic acid (cis-butenedioic acid) using a catalyst such as vanadium pentoxide (V2O5) or a combination of vanadium and molybdenum oxides. This step often involves high temperatures (around 300-350°C) and a gas-phase reaction.
- Maleic acid, in the presence of a catalyst, is then dehydrated to form maleic anhydride. Common catalysts include metal oxides such as vanadium pentoxide or mixed metal oxides like titanium-vanadium mixed oxides.
Butane Oxidation (n-Butane)
- This method involves the oxidation of n-butane, a four-carbon alkane, to maleic anhydride. This process also proceeds via a multi-step reaction sequence.
- In the first step, n-butane is oxidized to maleic acid, similar to the benzene oxidation route.
- Maleic acid is then dehydrated to yield maleic anhydride, typically using the same types of catalysts as in the benzene oxidation process.
Purification
- After the maleic anhydride is formed, it is usually purified through processes such as distillation or crystallization to remove any impurities and obtain the desired product in high purity.
Uses of Maleic Anhydride
- Raw material in the production of unsaturated polyester resins (UPRs). These resins are then used in composite materials, such as fiberglass-reinforced plastics (FRP), for applications including automotive parts, pipes, tanks, and construction materials.
- It is used in the synthesis of alkyd resins, which are important components in paints, coatings, and varnishes. Alkyd resins provide durability, gloss, and adhesion to the coatings.
- Can be hydrolyzed to produce fumaric acid, which has many applications in the food and beverage industry as an acidulant, flavor enhancer, and preservative. It is also used in the production of resins, polymers, and pharmaceuticals.
- Maleic anhydride derivatives are used in the production of certain herbicides, insecticides, and fungicides.
- Maleic anhydride polymers, such as poly(maleic anhydride-alt-1-octadecene), are used as scale and corrosion inhibitors in water treatment applications, particularly in cooling water systems.
- It is used in the production of textile auxiliaries, such as sizing agents and dyeing assistants.
Major producers
Source: Kirk & Othmer
| Company | Location | Capacity (KMT/Year) |
| Yongsan Chemicals, Inc. | South Korea | 38 |
| Bartek Ingredients Inc. | Canada | 28 |
| Sasol-Huntsman | Germany | 105 |
| DSM NV | The Netherlands | 100 |
| INEOS | USA | 50 |
| Huntsman Corporation | USA | 155 |
| Huntsman Performance Products | USA | 100 |
| Lanxess Corporation | USA | 75 |
| Lonza Group AG | Switzerland | 100 |
| AOC Materials | USA | 55 |
| Mitsubishi Chemical Corporation | Japan | 32 |
| Mitsui Chemicals, Inc | Japan | 33 |
| Mitsui Chemicals Polyurethanes, Inc. | Japan | 100 |
| Nippon Shokubai Co., Ltd | Japan | 35 |
| NOF Corporation | Japan | 12 |
| Polynt SpA | Italy | 96 |
| Mysore Petro Chemicals Ltd. | India | 15 |