Sodium thiosulfate, also known as hyposulfite or simply “hypo”, is an inorganic compound with the chemical formula Na₂S₂O₃. It commonly occurs as a pentahydrate (Na₂S₂O₃·5H₂O), which forms colorless, transparent crystals that are soluble in water. The compound is widely used in laboratories, photography, medicine, and water treatment. Sodium thiosulfate is best known for its ability to react with halogens like iodine, making it a standard reagent in iodometric titrations.
Structure
The molecular structure of sodium thiosulfate consists of:
- Two sodium (Na⁺) ions and one thiosulfate (S₂O₃²⁻) anion.
- The thiosulfate ion has a central sulfur atom bonded to three oxygen atoms (one double bond and two single bonds) and another terminal sulfur atom linked by an S–S bond.
- The geometry around the central sulfur is tetrahedral.
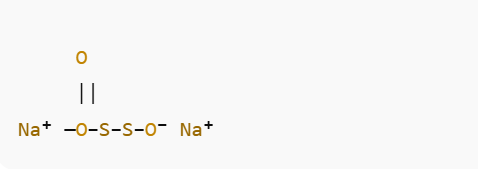
This arrangement shows the delocalized negative charge on the oxygen atoms and the S–S linkage that gives the ion its unique chemical reactivity.
Physical Properties
- Appearance: Colorless, crystalline solid (usually as pentahydrate).
- Molar mass:
- Anhydrous form: 158.11 g/mol
- Pentahydrate form: 248.18 g/mol
- Solubility: Highly soluble in water; insoluble in alcohol.
- Melting point: Around 48 °C (for pentahydrate; it loses water on heating).
- Taste: Slightly salty and cooling (though not safe for tasting).
- Density: 1.67 g/cm³ (pentahydrate).
- Decomposition: Decomposes on heating above 100 °C, forming sodium sulfate (Na₂SO₄), sulfur, and sulfur dioxide (SO₂).
Chemical Properties
Reaction with Acids:
Sodium thiosulfate reacts with acids (like HCl) to produce sulfur, sulfur dioxide, and water: Na2S2O3+2HCl→2NaCl+SO2+S↓+H2O. This reaction demonstrates its reducing properties and results in the characteristic milky appearance due to sulfur precipitation.
Reaction with Iodine:
Sodium thiosulfate reduces iodine to iodide, forming sodium tetrathionate: 2Na2S2O3+I2→Na2S4O6+2NaI. This is the basis of iodometric titrations, used to determine iodine concentration.
Reaction with Chlorine
Sodium thiosulfate reacts with chlorine to form sodium chloride and sodium sulfate: Na2S2O3+4Cl2+5H2O→2NaHSO4+8HCl. This reaction explains its use as a chlorine neutralizer in water treatment.
Oxidation
When oxidized by agents such as nitric acid, sodium thiosulfate forms sodium sulfate: Na2S2O3+2HNO3→2NaNO3+H2O+SO2+S
Preparation
Sodium thiosulfate can be prepared by several methods:
From Sodium Sulfite and Sulfur:
Na2SO3+S→Na2S2O3. This is the most common laboratory method, achieved by boiling a solution of sodium sulfite with excess sulfur.
From Sodium Hydroxide and Sulfur Dioxide:
6NaOH+4S+SO2→2Na2S2O3+2H2O+Na2SO3
As a By-product:
It can also be obtained as a by-product in certain industrial processes like the manufacture of sodium sulfite.
Uses
- In Photography:
Sodium thiosulfate acts as a fixing agent to dissolve unreacted silver halides from photographic films and papers. - In Medicine:
- Used as an antidote for cyanide poisoning, as it provides sulfur for the conversion of cyanide to thiocyanate (a less toxic compound).
- Sometimes used to treat calciphylaxis and certain dermatological conditions.
- In Analytical Chemistry:
- Widely used in iodometric titrations to determine the concentration of oxidizing agents (e.g., chlorine, iodine).
- In Water Treatment:
- Used to neutralize chlorine in pools and aquariums.
- In Textile and Leather Industry:
- Acts as a bleaching agent and a component in tanning processes.
- In Gold Extraction:
- Serves as an alternative lixiviant to cyanide for extracting gold from ores.