Sodium phosphate is an inorganic salt composed of sodium (Na⁺) ions and phosphate (PO₄³⁻) ions, with the chemical formula Na₃PO₄. It is commonly available in anhydrous form as a white crystalline solid or as a hydrated form (commonly Na₃PO₄·12H₂O), which appears as colorless, deliquescent crystals.
Sodium phosphate is highly soluble in water, forming an alkaline solution due to hydrolysis of the phosphate ions. It is widely used in water treatment, detergents, food additives, and laboratory applications because of its buffering, sequestering, and pH-regulating abilities.
Structure
Sodium phosphate consists of:
- Three sodium ions (Na⁺)
- One phosphate ion (PO₄³⁻)
The phosphate ion is a tetrahedral polyatomic ion, with a central phosphorus atom covalently bonded to four oxygen atoms. One oxygen atom carries a full negative charge, while the others share negative charges due to resonance:
Tetrahedral structure of PO₄³⁻:
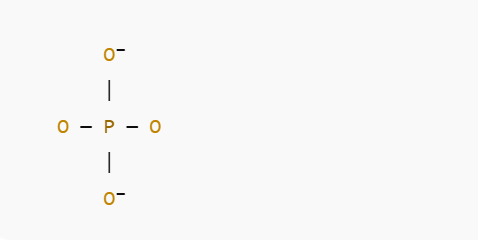
The Na⁺ ions are ionically bonded to the negatively charged oxygen atoms of the phosphate ion.
In the solid state, this forms a three-dimensional ionic lattice, contributing to the stability and high melting point of sodium phosphate.
Physical Properties
- Appearance:
White crystalline solid in anhydrous form; colorless deliquescent crystals in hydrated form (commonly Na₃PO₄·12H₂O). - Molecular Weight:
- Anhydrous: 163.94 g/mol
- Dodecahydrate: 380.12 g/mol
- Solubility:
Highly soluble in water, forming a strongly alkaline solution. Practically insoluble in alcohol and ether. - Melting Point:
- Anhydrous: ~ 1580 °C
- Hydrate: Loses water and decomposes at lower temperatures.
- Density:
Approximately 2.54 g/cm³ (anhydrous form). - Odor and Taste:
Tasteless, odorless, but forms an alkaline solution in water. - Hygroscopic Nature:
Hydrated forms are deliquescent, absorbing moisture from air.
Chemical Properties
Alkalinity:
Sodium phosphate is a strong base in aqueous solution due to hydrolysis of PO₄³⁻ ions:
PO43−+H2O⇌HPO42−+OH−
Reaction with Acids:
Reacts with acids to form phosphoric acid or its salts:
Na3PO4+HCl→NaCl+Na2HPO4
Buffering Ability:
Forms phosphate buffer solutions when mixed with dihydrogen phosphate (NaH₂PO₄) or hydrogen phosphate (Na₂HPO₄), maintaining pH stability.
Reaction with Metal Ions:
Can react with calcium, magnesium, or iron ions to form insoluble phosphate precipitates:
3Ca2++2PO43−→Ca3(PO4)2↓
Thermal Stability:
- Stable at room temperature.
- At very high temperatures, decomposes into sodium pyrophosphate (Na₄P₂O₇) and releases oxygen.
Preparation
From Sodium Carbonate and Phosphoric Acid:
3Na2CO3+2H3PO4→2Na3PO4+3CO2↑+3H2O
Sodium carbonate reacts with phosphoric acid, releasing carbon dioxide and water, forming sodium phosphate.
From Sodium Hydroxide and Phosphoric Acid:
3NaOH+H3PO4→Na3PO4+3H2O
A neutralization reaction between a strong base and a triprotic acid.
Industrial Preparation:
- Often produced from triple superphosphate or phosphate rock by reacting with sodium carbonate or sodium hydroxide.
Uses of Sodium Phosphate (Na₃PO₄)
Water Treatment:
Used to soften water and prevent the formation of scale by sequestering calcium and magnesium ions.
Detergents and Cleaning Agents:
Acts as a builder in detergents, enhancing cleaning efficiency and helping to remove grease and stains.
Food Additive:
Used as a food preservative (E339), pH regulator, and emulsifier in processed foods like cheese, canned meat, and baked goods.
Laboratory Reagent:
Serves as a buffering agent and reagent in chemical experiments and analyses.
Dental Products:
Found in some toothpastes to prevent tartar formation and maintain oral pH.
Pharmaceuticals:
Used in laxatives and electrolyte solutions, providing phosphate ions for bodily functions.
Agriculture:
Serves as a source of phosphorus in fertilizers, promoting healthy plant growth.
Industrial Applications:
Employed in metal treatment, ceramics, and textile industries for pH control and as a dispersing agent.
Buffer Solutions:
Used in phosphate buffer solutions for biochemical and molecular biology experiments, maintaining stable pH.
Analytical Chemistry:
Forms precipitates with certain metal ions, which can be useful in qualitative and quantitative analyses of calcium, magnesium, and other metals.