Sulfur hexafluoride (SF₆) is a colorless, odorless, and non-flammable gas commonly used as an electrical insulator in high-voltage equipment such as transformers and circuit breakers. Chemically, it is one of the most stable and inert compounds known. To understand its exceptional stability, we can analyze its Lewis structure, which shows how sulfur and fluorine atoms share electrons.
Step 1: Count the Total Valence Electrons
To begin constructing the Lewis structure, we calculate the total number of valence electrons available in SF₆:
- Sulfur (S) belongs to Group 16 of the periodic table and thus has 6 valence electrons.
- Fluorine (F) belongs to Group 17, with 7 valence electrons each. Since there are six fluorine atoms, they contribute 6 × 7 = 42 valence electrons.
Adding these together, the total number of valence electrons is:
6 (S) + 42 (F) = 48 valence electrons.
These electrons will be distributed among the atoms to form bonds and lone pairs in the molecule.
Step 2: Determine the Central Atom
The central atom in the molecule is the atom that can form the most bonds or is least electronegative. In SF₆, sulfur serves as the central atom because it can expand its octet (use d orbitals to accommodate more than eight electrons) — something that fluorine cannot do. Each fluorine atom will surround sulfur to create a symmetrical structure.
Step 3: Connect Atoms with Single Bonds
Next, each fluorine atom is connected to the central sulfur atom by a single covalent bond (S–F). Since there are six fluorine atoms, we use 6 single bonds, which consume 12 electrons (6 bonds × 2 electrons). After forming these bonds, each fluorine still needs to complete its octet, and we have 48 – 12 = 36 electrons remaining to distribute.
Step 4: Complete the Octets of Fluorine Atoms
Each fluorine atom already has 2 bonding electrons from its bond with sulfur, and needs 6 more to complete its octet. Therefore, each fluorine gets 3 lone pairs (6 electrons).
Since there are six fluorine atoms, 6 × 6 = 36 electrons are used to complete their octets — exactly the number we have left. Thus, all 48 valence electrons have been used appropriately.
Step 5: Verify the Octet and Expanded Octet
At this point:
- Each fluorine atom has a complete octet (8 electrons total: 2 shared + 6 lone).
- The sulfur atom, however, now has 12 electrons around it — one pair from each of the six S–F bonds. This is an example of an expanded octet, which is allowed for elements in period 3 or higher (sulfur, phosphorus, etc.) because they have available d orbitals that can participate in bonding.
This satisfies all atoms, and the Lewis structure is complete and stable.
Step 6: Final Lewis Structure Description
In the Lewis structure of SF₆, the sulfur atom is at the center, surrounded by six fluorine atoms arranged symmetrically. Each S–F bond is a single bond, and each fluorine has three lone pairs. There are no lone pairs on sulfur in this structure.
A text representation looks like this:
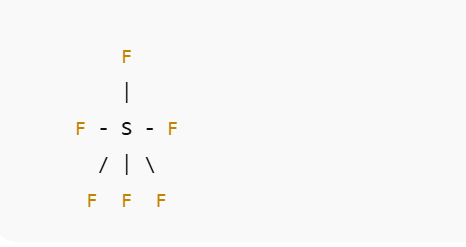
Each fluorine atom also carries three lone pairs of electrons (not shown here).
Molecular Geometry of SF₆
According to the VSEPR theory (Valence Shell Electron Pair Repulsion theory), the molecular geometry of SF₆ is octahedral. This is because there are six bonding pairs and no lone pairs around the central sulfur atom. The electron pairs repel each other equally, positioning the six fluorine atoms as far apart as possible — at the corners of an octahedron.
This symmetrical arrangement makes SF₆ a nonpolar molecule, even though each S–F bond is polar, because the bond dipoles cancel out due to the molecule’s symmetry.
Bond Angle in SF₆
In an octahedral molecular geometry, all the bond angles between the S–F bonds are 90°. The fluorine atoms are positioned symmetrically — four in one plane (forming a square) and one above and one below that plane. Thus, every fluorine atom is equidistant from the central sulfur atom, contributing to the molecule’s remarkable stability.
Hybridization of the Central Atom (Sulfur)
To accommodate six bonding pairs, the central sulfur atom undergoes sp³d² hybridization.
Here’s how this happens:
- One s orbital, three p orbitals, and two d orbitals from sulfur’s valence shell mix to form six equivalent sp³d² hybrid orbitals.
- Each of these hybrid orbitals overlaps with a fluorine atom’s 2p orbital to form a sigma (σ) bond.
This hybridization allows sulfur to form six equivalent S–F bonds arranged in an octahedral geometry.
Summary Table
| Property | Description |
|---|---|
| Molecular formula | SF₆ |
| Central atom | Sulfur (S) |
| Total valence electrons | 48 |
| Bonds | 6 single S–F bonds |
| Lone pairs on sulfur | 0 |
| Lone pairs on each fluorine | 3 |
| Molecular geometry | Octahedral |
| Bond angle | 90° and 180° |
| Polarity | Nonpolar molecule |
| Type of molecule | Hypervalent (expanded octet) |