Magnesium hypochlorite is an inorganic compound with the chemical formula Mg(OCl)₂. It is the magnesium salt of hypochlorous acid (HClO) and belongs to the family of metal hypochlorites, which are known for their strong oxidizing and disinfecting properties.
In its pure form, magnesium hypochlorite appears as a white crystalline solid or powder with a chlorine-like odor due to the slow release of chlorine gas. It is highly unstable when heated or exposed to light, as it decomposes to form magnesium chloride (MgCl₂) and oxygen or chlorine gas.
Magnesium hypochlorite is used as a disinfectant, bleaching agent, and water purifier, similar to calcium hypochlorite. It is valued for its bactericidal and deodorizing properties, particularly in applications where calcium buildup (from Ca(OCl)₂) is undesirable.
Structure
The structure of magnesium hypochlorite consists of:
- One magnesium ion (Mg²⁺)
- Two hypochlorite ions (OCl⁻)
Each hypochlorite ion is composed of one oxygen atom (O) covalently bonded to one chlorine atom (Cl), carrying an overall –1 charge.
Ionic Structure Representation:
Mg2++2(OCl−)
Expanded Structural Diagram:
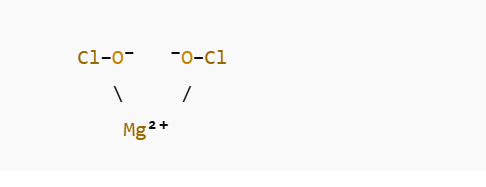
In the solid lattice:
- The Mg²⁺ cation is surrounded by OCl⁻ anions through ionic bonding.
- The O–Cl bond within the hypochlorite ion is polar covalent, while the attraction between Mg²⁺ and OCl⁻ is ionic.
- The structure forms a crystalline ionic network that is relatively stable when dry but decomposes in moisture or heat.
Physical Properties
- Appearance:
White crystalline solid or fine powder with a chlorine-like odor. - Molecular Weight:
146.21 g/mol - Solubility:
- Soluble in water, forming a pale yellow solution that slowly decomposes, releasing chlorine and oxygen.
- Insoluble in most organic solvents.
- Odor:
Has a strong, pungent chlorine smell due to slow release of chlorine gas. - Stability:
- Unstable when exposed to heat, light, or moisture.
- Decomposes on standing, giving off oxygen or chlorine gas and forming magnesium chloride (MgCl₂).
- Density:
Approximately 2.18 g/cm³ (estimated for anhydrous form). - Color:
White to off-white crystalline appearance. - Melting Point:
Decomposes before melting, usually around 170–200°C.
Chemical Properties
Decomposition:
Magnesium hypochlorite easily decomposes when heated or exposed to light: 2Mg(OCl)2→2MgCl2+O2↑ or, under moist conditions: Mg(OCl)2→MgCl2+O2↑+Cl2↑ This shows its strong oxidizing nature.
Reaction with Water:
Dissolves in water forming hypochlorous acid (HClO), which is a strong oxidizing and disinfecting agent: Mg(OCl)2+2H2O→2HClO+Mg(OH)2
Reaction with Acids:
Reacts vigorously with acids, liberating chlorine gas: Mg(OCl)2+2HCl→MgCl2+Cl2↑+H2O. This is a characteristic reaction of hypochlorites.
Oxidizing Property:
Acts as a strong oxidizing agent, oxidizing organic compounds and certain metal ions.
For example, it can oxidize ferrous ions (Fe²⁺) to ferric ions (Fe³⁺): 2Fe2++Mg(OCl)2+2H+→2Fe3++Mg2++2Cl−+H2O2
Bleaching Action:
The nascent oxygen (O) or hypochlorous acid produced from its decomposition has powerful bleaching and disinfecting effects.
Preparation
Magnesium hypochlorite can be prepared by several laboratory and industrial methods:
From Magnesium Hydroxide and Chlorine Gas:
Mg(OH)2+Cl2→Mg(OCl)2+H2O
- This reaction occurs in cold aqueous solution.
- The magnesium hydroxide reacts with chlorine gas, forming magnesium hypochlorite and water.
- The reaction must be controlled to prevent over-chlorination and decomposition.
From Magnesium Carbonate and Hypochlorous Acid:
MgCO3+2HClO→Mg(OCl)2+H2O+CO2↑
- Hypochlorous acid reacts with magnesium carbonate to yield magnesium hypochlorite, with carbon dioxide as a by-product.
From Magnesium Chloride and Calcium Hypochlorite (Double Decomposition): MgCl2+Ca(OCl)2→Mg(OCl)2+CaCl2
- A metathesis reaction, where calcium hypochlorite transfers hypochlorite ions to magnesium.
- The reaction is typically carried out in aqueous medium under controlled temperature.
Electrochemical Method:
- Magnesium hypochlorite can also be formed electrolytically by passing current through a magnesium chloride solution, where chlorine generated at the anode reacts with magnesium hydroxide at the cathode to produce Mg(OCl)₂.
Uses of Magnesium Hypochlorite (Mg(OCl)₂)
Water Disinfection:
Used to purify drinking water and sanitize swimming pools, killing bacteria, viruses, and algae through the release of hypochlorous acid (HClO).
Bleaching Agent:
Acts as a bleaching chemical in the textile and paper industries, where it removes stains and whitens fabrics and paper pulp.
Industrial Sanitizer:
Employed as a surface disinfectant in food processing plants, breweries, and dairy industries to maintain hygiene and prevent microbial growth.
Wastewater Treatment:
Used in sewage and industrial wastewater treatment to disinfect effluents and control odor by oxidizing organic contaminants.
Disinfectant for Public Facilities:
Commonly applied in hospitals, public toilets, and sanitation facilities to disinfect surfaces, instruments, and environments.
Agricultural Sanitizer:
Used to sterilize irrigation water, clean farm equipment, and control microbial contamination in greenhouses and food storage areas.
Deodorizing Agent:
Helps remove or neutralize unpleasant odors caused by bacterial decomposition, especially in waste containers or sewage systems.
Oxidizing Agent in Chemical Processes:
Serves as a mild oxidizing agent in certain organic and inorganic reactions, where controlled oxidation is required.
Oral and Medical Disinfection:
In diluted form, can be used in the formulation of antiseptic rinses and medical-grade disinfectants, though this requires strict control to prevent irritation.
Laboratory Use:
Utilized in laboratories for chlorination reactions, sterilization of instruments, and preparation of disinfectant solutions for research and testing.