A Lewis structure, or Lewis dot structure, is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist. These diagrams represent the valence electrons as dots, with lines connecting atoms to show shared electron pairs (covalent bonds). Drawing a Lewis structure involves several steps, including calculating total valence electrons, identifying the central atom, and arranging electrons as bonds and lone pairs until all atoms have a stable electron configuration, usually an octet (or duet for hydrogen).
Lewis Structure of the Nitrite Ion (NO₂⁻) — step by step, covering its structure, bonding, resonance, and geometry.
Basic Information
- Formula: NO₂⁻
- Name: Nitrite ion
- Total atoms: 1 Nitrogen (N), 2 Oxygen (O)
- Overall charge: -1
Step 1: Count Total Valence Electrons
Each atom contributes valence electrons according to its group number:
- Nitrogen (Group 15): 5 valence electrons
- Oxygen (Group 16): 6 valence electrons × 2 oxygens = 12
- Extra electron (because of the -1 charge): +1
5+12+1=18 total valence electrons
So, NO₂⁻ has 18 valence electrons to distribute.
Step 2: Determine the Central Atom
- Nitrogen is less electronegative than oxygen, so N becomes the central atom.
- Thus, the skeleton structure is:
O–N–O
Step 3: Place Single Bonds
Draw single bonds (σ-bonds) between nitrogen and each oxygen.
Each N–O single bond uses 2 electrons × 2 = 4 electrons.
Remaining electrons:
18−4=14 electrons left
Step 4: Complete Octets on Outer Atoms (Oxygens)
Each oxygen needs 8 electrons (including bonding pairs):
- Each O already has 2 electrons from its bond with N.
- To complete each O’s octet, give 6 more electrons (3 lone pairs each).
Two oxygens × 6 = 12 electrons used.
Remaining electrons:
14−12=2 electrons left
These 2 remaining electrons go on nitrogen as a lone pair.
Step 5: Check Octets
Now nitrogen has:
- 2 single bonds = 4 bonding electrons
- 1 lone pair = 2 nonbonding electrons
→ Total = 6 electrons (not a full octet) - So, one of the oxygens should form a double bond with nitrogen to complete N’s octet.
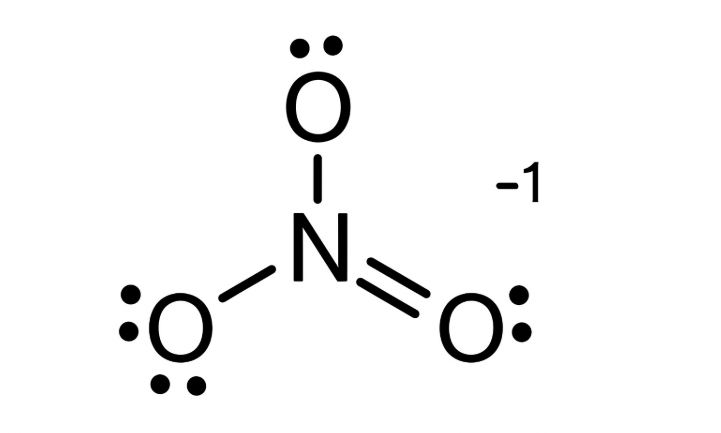
Step 6: Form a Double Bond
Convert one lone pair from an oxygen atom into a bonding pair with nitrogen:
O=N−O
Now nitrogen has:
- 1 double bond (4 electrons)
- 1 single bond (2 electrons)
- 1 lone pair (2 electrons)
→ Total = 8 electrons → octet satisfied.
Step 7: Check Formal Charges
Let’s assign formal charges for each atom.
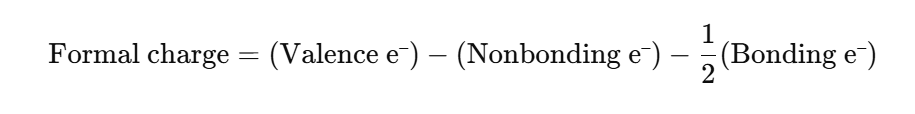
Case 1 — Structure with one double bond:
For Nitrogen (N):
- Valence = 5
- Nonbonding = 2
- Bonding = 6 (1 double bond + 1 single bond = 3 bonds = 6 shared e⁻)
→ FC = 5 – 2 – 3 = 0
For Double-bonded Oxygen (O):
- Valence = 6
- Nonbonding = 4
- Bonding = 4
→ FC = 6 – 4 – 2 = 0
For Single-bonded Oxygen (O⁻):
- Valence = 6
- Nonbonding = 6
- Bonding = 2
→ FC = 6 – 6 – 1 = -1
Sum of formal charges = 0 + 0 + (-1) = -1, matches the ion’s charge.
Step 8: Resonance Structures
There are two equivalent resonance structures for NO₂⁻:
- One where the left oxygen has the double bond.
- One where the right oxygen has the double bond.
They can be represented as:
[O=N−O]−↔[O−N=O]−
Each oxygen alternately carries the negative charge.
Thus, the true structure is a resonance hybrid, meaning the N–O bonds are equivalent and intermediate between a single and double bond.
Step 9: Bond Order
Since there are two resonance structures and three N–O bonds (2 total double-bond electrons shared over both N–O bonds), the average bond order is:
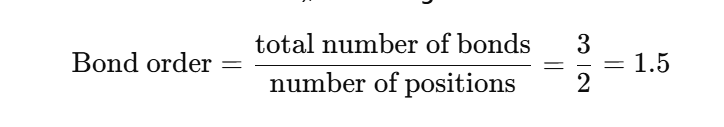
So, each N–O bond has a bond order of 1.5.
Step 10: Molecular Geometry (VSEPR Theory)
- Central atom: Nitrogen
- Steric number = 3 (2 bonding regions + 1 lone pair)
→ Electron geometry: Trigonal planar
→ Molecular shape: Bent (angular)
Bond angle: approximately 115–120°
Step 11: Summary of Key Features
| Property | Description |
|---|---|
| Chemical Formula | NO₂⁻ |
| Total Valence Electrons | 18 |
| Resonance Structures | 2 equivalent |
| Bond Order | 1.5 |
| Shape | Bent |
| Electron Geometry | Trigonal planar |
| Bond Angle | ~115–120° |
| Hybridization | sp² on nitrogen |
| Formal Charge Distribution | One O = -1, N = 0, other O = 0 |
Final Representation:
\ce[O=N–O]−↔\ce[O–N=O]−
Lewis structure with dots and bonds