Ethyne (C₂H₂), commonly known as acetylene, is the simplest alkyne—a hydrocarbon containing a carbon–carbon triple bond. It is a colorless, flammable gas used in welding, cutting metals, and as a chemical building block in industry. Understanding its Lewis structure helps explain why acetylene is so reactive and has strong C–C bonding.
Step 1: Determine Total Number of Valence Electrons
Each atom contributes the following valence electrons:
- Carbon (C) → 4 valence electrons × 2 = 8
- Hydrogen (H) → 1 valence electron × 2 = 2
Total valence electrons:
8+2=10 valence electrons
These 10 electrons will be arranged to form stable bonds.
Step 2: Arrange the Atoms
Hydrogen atoms can only form one bond each, so they must be attached to the carbon atoms.
The two carbons will bond with each other in the center.
So the skeletal structure is:
H–C–C–H
Step 3: Form Single Bonds
Each single bond uses 2 electrons:
- Two C–H bonds = 4 electrons
- One C–C bond = 2 electrons
So far, 6 electrons are used, leaving:
10−6=4 electrons remaining
Step 4: Complete the Octets
Currently:
- Each carbon has 4 electrons (2 from C–H and 2 from C–C).
- Each carbon needs 8 electrons to satisfy the octet rule.
We have 4 electrons left, which can form two more bonds between the carbon atoms.
That gives us a triple bond (one sigma + two pi bonds) between the two carbons.
Step 5: Verify the Structure
Now, the structure is:
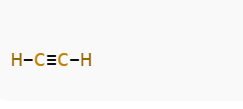
Let’s check electron counts:
- Each C–H bond = 2 electrons → 4 total
- The C≡C bond = 6 electrons
Total = 10 electrons
Each carbon atom has 8 electrons around it (octet satisfied):
- 2 from its bond with hydrogen
- 6 from the triple bond with the other carbon
Each hydrogen has 2 electrons (duet satisfied).
Lewis Structure Diagrammatically
Here’s the Lewis structure with dots and bonds:
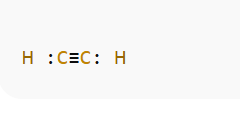
Step 6: Molecular Geometry
According to VSEPR theory, each carbon is surrounded by two regions of electron density (one triple bond and one single bond), which gives a linear molecular geometry.
- Bond angle: 180°
- Shape: Linear
- Hybridization of each carbon: sp hybridized
Step 7: Polarity
- The molecule is nonpolar overall, because the structure is symmetrical and dipoles cancel each other out.
- However, each C–H bond is slightly polar due to the difference in electronegativity between C and H.
Summary Table
| Property | Description |
|---|---|
| Molecular formula | C₂H₂ |
| Common name | Acetylene |
| Total valence electrons | 10 |
| Central atoms | 2 Carbon atoms |
| Bonds | 1 C≡C triple bond, 2 C–H single bonds |
| Lone pairs | None |
| Geometry | Linear |
| Bond angle | 180° |
| Hybridization (C) | sp |
| Polarity | Nonpolar molecule |