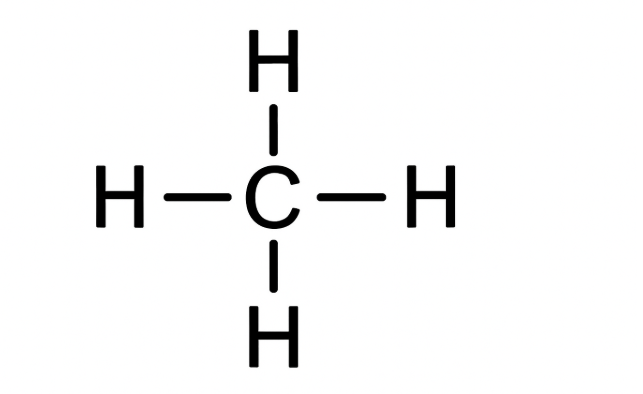
A Lewis structure, or Lewis dot structure, is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist. These diagrams represent the valence electrons as dots, with lines connecting atoms to show shared electron pairs (covalent bonds). Drawing a Lewis structure involves several steps, including calculating total valence electrons, identifying the central atom, and arranging electrons as bonds and lone pairs until all atoms have a stable electron configuration, usually an octet (or duet for hydrogen).
Basic Information
- Chemical formula: CH₄
- Compound name: Methane
- Atoms involved: 1 Carbon (C) and 4 Hydrogen (H) atoms
- Type: Covalent compound (nonpolar)
Step 1: Count Total Valence Electrons
Each atom contributes its valence electrons:
- Carbon (Group 14) → 4 valence electrons
- Hydrogen (Group 1) → 1 valence electron × 4 H = 4 electrons
Total valence electrons = 4 (C) + 4 (H) = 8 electrons
So, we have 8 valence electrons to distribute in the Lewis structure.
Step 2: Determine the Central Atom
- Carbon is less electronegative than hydrogen (and can form multiple bonds), so it becomes the central atom.
- Hydrogens surround it.
Basic skeleton:
H – C – H
Step 3: Form Single Bonds
Each C–H single bond uses 2 electrons (one shared pair).
4 bonds × 2 electrons = 8 electrons used.
That’s all 8 electrons — meaning every valence electron is used in bonding.
Step 4: Check Octets and Duets
- Hydrogen atoms: Each has 2 electrons (1 bond) → full duet (stable for H).
- Carbon atom: Has 4 bonds × 2 = 8 electrons → full octet.
Step 5: Verify Formal Charges
Formal charge formula:
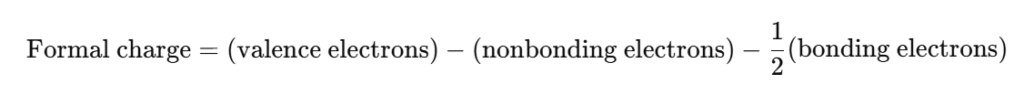
- Carbon: 4 – 0 – (8 ÷ 2) = 0
- Each Hydrogen: 1 – 0 – (2 ÷ 2) = 0
→ All formal charges are zero, confirming the structure is stable.
Step 6: Geometry (VSEPR Theory)
- Central atom: Carbon
- Bonding pairs: 4 (C–H bonds)
- Lone pairs on carbon: 0
According to VSEPR theory (AX₄ type):
- Electron geometry = Tetrahedral
- Molecular geometry = Tetrahedral
Step 7: Bond Angles and Hybridization
- Bond angle: 109.5° (ideal tetrahedral angle)
- Hybridization of carbon: sp³
Each sp³ orbital overlaps with an H 1s orbital, forming four sigma (σ) bonds.
Step 8: Polarity
- Each C–H bond is slightly polar due to the small electronegativity difference between C and H.
- However, the tetrahedral symmetry causes the dipoles to cancel.
Thus, CH₄ is a nonpolar molecule overall.
Step 9: Summary of Key Features
| Property | Description |
|---|---|
| Formula | CH₄ |
| Total Valence Electrons | 8 |
| Bond Type | 4 single covalent (σ) bonds |
| Central Atom | Carbon |
| Shape | Tetrahedral |
| Bond Angle | 109.5° |
| Hybridization | sp³ |
| Polarity | Nonpolar |
| Formal Charges | All 0 |
Lewis structure with dots and bonds